Impurity Adsorption Studies for Alioth Alidep® Depth Filters(II)
Introduction
In the previous article, we studied the impurity adsorption capabilities of Alioth depth filters for HCP (Host Cell Proteins) and DNA removal. Today, we will take a closer look at HCP and DNA, two process-related impurities that are unavoidable in biopharmaceuticals. We will also discuss Protein A ligands, a process-related impurity that sheds during downstream purification after affinity chromatography.
HCP includes secretory proteins and intracellular proteins, the latter of which can be released from apoptotic dead cells during the production culture process or harvesting steps. Excessive HCP content may trigger an immune response in the human body. HCP content is detected using the ELISA analytical method with commercially available host cell protein detection kits. Residual host cell DNA may carry tumor- or virus-related genes, posing potential risks. Therefore, regulatory agencies worldwide have strict limits on its residual levels. DNA content is detected using Quantitative Fluorescence PCR (QF-PCR) with commercially available DNA detection kits.
Residual Protein A is a common impurity introduced during the antibody purification process. The antibody stock solutions are purified through Protein A chromatography. during which various factors such as process conditions, pH, or additives, as well as proteases in the culture supernatant, may degrade Protein A and disrupt its coupling with the chromatographic medium. As a result, a small amount of Protein A may detach from the chromatographic medium and become an impurity in the final product. Excessive intake of Protein A in the human body may trigger immune reactions and other potential risks, making it necessary to monitor its residual levels. Protein A content is detected using the ELISA analytical method with commercially available protein detection kits. The detection results are used to calculate the log reduction factor (LRF) at each step to assess the process's ability to remove these three major
impurities.
How does depth filtration remove these impurities? Let's review the interception mechanism of depth filtration. In depth filters, particle removal occurs both on the surface and within the filtration matrix. The removal efficiency is determined by the depth and tortuosity of the flow channels, as well as the size of the particles. These characteristics allow depth filters to have a very high capacity for particle removal. Most particles naturally carry a negative charge, so using positively charged modified filtration media effectively removes smaller particles. Charge modification enables the filter to efficiently remove particles and colloids without significantly impacting capacity or pressure, Additionally, it effectively removesnegatively charged impurities, such as host cell proteins (HCP) and host cell DNA generated during fermentation fluid cell culture, as mentioned earlier.
Materials and methods
The purpose of this study was to evaluate the performance of Alioth depth filter and M product in removing DNA & HCP impurities and protein A during mAb purification. The feed solutions usedinclude post-fermentation cell culture fluid and fluid after low-pH incubation following affinity chromatography.
Case study 1:Depth filtration of cell culture fluid after centrifugation
Table 1. The feed solution information

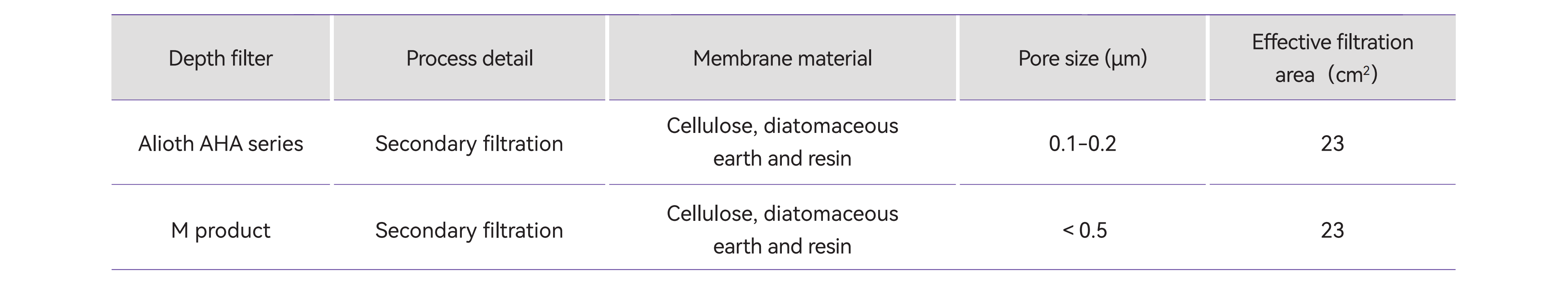
Table 2. Test filters Information
Results
The removal efficiency of DNA and HCP in cell culture fluid with depth filtration can be assessed by measuring the residual amount of DNA & HCP in the filtrate solution and calculating corresponding log reduction value (LRV).
Table 3 summarizes the performance of Alioth AHA depth filter and M product, respectively. The data showed that Alioth AHA filter outperformed M product in terms of throughput, post-filtration endpoint differential pressure and turbidity control.
Table 3. Testing result


Figure 1 and Figure 2 show that the DNA & HCP removal performance of Alioth AHA series depth filter and M product with same volume of culture fluid samples. The DNA residue (pg/mL) & HCP residue (ng/mL) in the fluid filtrated by Alioth AHA series filter is lower than that of M product and Alioth AHA series have higher LRV of DNA & HCP removal capabilities than M product, indicating that the Alioth AHA series filters have better performance in DNA & HCP removal.
Result analysis
Under conditions where the Alioth AHA series depth filters outperform the M filter in terms of loading capacity, final differential pressure, and filtrate turbidity, the residual levels of DNA and HCP after filtration remain significantly lower than those with the M filter.
As a post centrifugation clarification step, the Alioth AHA series depth filters demonstrate superior performance in both turbidity control of the filtrate and impurity adsorption capacity.
Case study 2: Depth filtration of mAb after low pH incubation following affinity chromatography
Table 4. The feed solution information

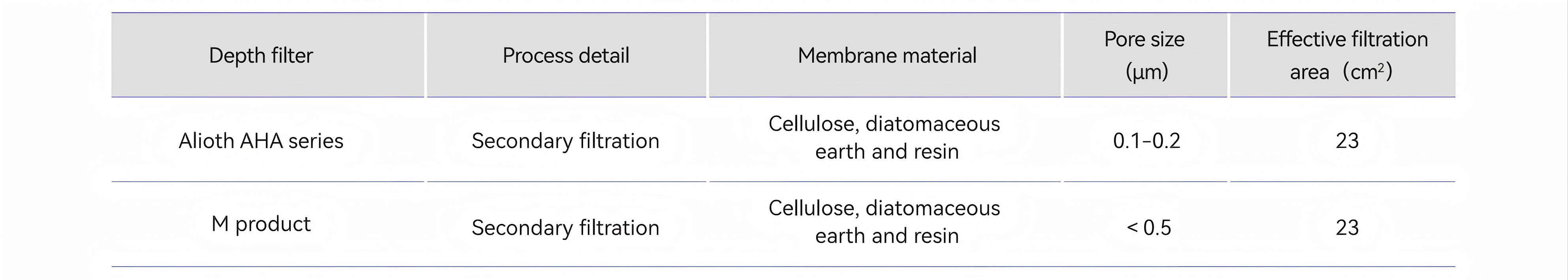
Table 5. Test filters Information
Results
The removal efficiency of DNA & HCP & Protein A in fluid by depth filtration after low-pH incubation can be assessed by measuring the residual amount of DNA & HCP & protein A in the filtrate solution and calculating corresponding LRV.
Table 6 summarizes the impurity removal performance of DNA and HCP in post-affinity chromatography low-pH incubation feed solution at a loading of 1000 g/m² after filtration through different filters (Alioth AHA series filters and M filters). The data indicate that the Alioth AHA series filters outperform the M filter in terms of loading capacity, final differential pressure, and filtrate turbidity.
Table 6. Testing result


Figure 3 and Figure 4 show that the DNA & HCP & Protein A removal performance of Alioth AHA series depth filters and M product with same loading capacity (1000 g/m2) of fluid by depth filtration after low-pH incubation following affinity chromatography.
The DNA residue (pg/mL) & HCP residue (ng/mL) & Protein A residue (pg/mL) in the fluid filtrated by Alioth AHA series filters are lower than that of M product and Alioth AHA series have higher LRV of DNA & HCP & Protein A removal than M product, indicating that the Alioth AHA series filters have better performance in DNA & HCP & Protein A removal.
Result analysis
In the depth filtration process after low-pH incubation following affinity chromatography, Alioth AHA series depth filters outperformed M product in terms of log reduction in the removal of HCP & DNA & Protein A. Alioth AHA series depth filters showed superior performance in the adsorption of impurities.
Summary
Through two case studies focusing on the initial clarification of cell culture fluid and depth filtration after low-pH incubation following affinity chromatography, this study consistently validated the superior impurity removal performance of various Alioth depth filters compared to the widely used M filter under identical test conditions. The Alioth filters demonstrated stronger adsorption capabilities for HCP, DNA, and even residual Protein A, significantly reducing the burden on downstream purification processes!

